MTBE Contamination in Groundwater: Sources and Behavior

By Leigh Horton, staff writer for Save The Water™ | June 12, 2017
Introduction
This report synthesizes information about the effects of Methyl Tertiary Butyl Ether (MTBE) on groundwater and public drinking wells. It is based solely on a literature review of relevant materials from predominantly American organizations researching wells in the United States. This report is a data-based investigation of MTBE contamination.
Background
In the late 1970’s, MTBE was occasionally used as a gasoline additive to improve engine efficiency by increasing octane levels. The Clean Air Act Amendments introduced in 1990 to reduce smog and other pollutants resulted in an increased use of MTBE.4 These amendments did not require that oil companies add MTBE specifically, but economic consideration led most of them to add MTBE in their gasoline instead of alternative oxygenates. After these regulations were passed, groundwater readings began to reveal that MTBE exceeded the maximum contamination levels. In regions where MTBE contamination was the most noticeable, in states such as California and Colorado, residents began complaining about undrinkable water supplied by cities and private wells. Investigations were conducted by private and federal organizations. Their findings indicated that the additional MTBE usage was most likely contributing to water contamination. Their results contributed to the reduction of MTBE use in gasoline. In 2005, the US government no longer required reformulated gasoline to have oxygenates like MTBE, and state bans substantially reduced the amount of MTBE used.
Methods
This report is based on a literature review of sources from the Environmental Protection Agency and other government agencies and hearings. The sources referenced in this report are listed in the citations section. For the sake of brevity, not all the literature read is included in this report, only the sources referenced.
Findings
Major Sources and the Extent of MTBE in Groundwater
Benjamin H. Grumbles, the Deputy Assistant Administrator for Water for the EPA in 2002, stated that, “the most significant sources of contamination of water resources [from MTBE] are from leaking underground and aboveground storage tanks, pipelines, refueling spills, emissions from older marine engines, and to a much lesser degree, storm runoff and precipitation”.6 The United States Geological Survey, USGS, conducted research that concluded high concentrations of MTBE contamination come from leaking underground storage tanks, common structures used at gas stations or any other facility containing hazardous materials.6 The USGS also found that in their 31-state study, 9,000 wells may be within a one kilometer radius of a leaking underground storage tank.6 However, not all wells near the tanks are at risk of contamination. Contamination is determined by the direction of groundwater flow, geologic properties, and other factors.
Reported Health Problems
The most common complaint has been the taste-and-smell-quality of drinking water. The health effects are minimal but include respiratory issues near MTBE sources, headaches, coughing, burning throat or nose, eye irritation, nausea or vomiting, disorientation, and dizziness.1
MTBE Behavior in Groundwater
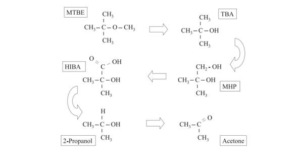
MTBE is highly soluble in water and in certain environments, it can biodegrade in groundwater and soil where sufficient oxygen is present and in bed sediments of streams, lakes, wetlands, and estuaries.6 However, this breakdown of organic contaminants by microorganisms when oxygen is present, aerobic biodegradation, turns MTBE to acetone (see Figure 1).
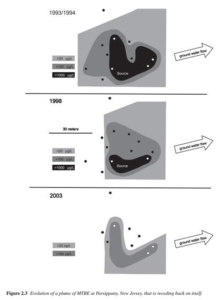
MTBE also moves readily with groundwater (see Figure 2). The EPA states that the length of the plume correlates to MTBE concentration. The higher the concentration, the larger the plume will be and vice versa. The plume length is difficult to predict after a spill because of the variability of MTBE concentration in gasoline.2 Initial concentrations of MTBE in the gasoline are not uniform. In other words, some gasoline may contain 11 percent MTBE (a standard value) but another batch of gasoline may only contain nine percent MTBE. Therefore, the plume from the gasoline containing 11 percent MTBE will be larger than that formed by the nine percent MTBE gasoline. Additional factors are characteristics of the aquifer through which the MTBE flows. The EPA states that “concentration of MTBE in a well can change through the combined influence of dilution and dispersion, biodegradation, sorption, and mixing of the contaminant plume with cleaner water in a monitoring well.” Their research also indicates that the addition of MTBE does not change the groundwater movement.2 Finally, the EPA indicates that, the size of a plume reflects a balance between the rate of release of the contaminant from the source area into the aquifer, the rate of transport of the contaminant away from the source area, and the rates of degradation and dispersion in groundwater which remove mass or reduce plume concentration.2
Discussion
Since groundwater can readily transport MTBE, many private and public wells have been increasingly contaminated since the Clean Air Act Amendments in 1990. Since 2005, the amount of MTBE added to gasoline has dramatically reduced because of state bans and federal regulations; although, past sources for MTBE are still problematic. Leaking underground and aboveground storage tanks should be regulated, especially those that still store gasoline enhanced or unenhanced with MTBE. Gasoline spills should be treated with care and studied to further determine how gasoline moves through aquifers. The health problems associated with exposure to MTBE are not life-threatening in most cases. Physical irritation and taste and smell discomfort from drinking water are generally the extents of the health problems.1 Since MTBE becomes acetone in groundwater after biodegradation, there is a concern of cellular damage and unconsciousness from the increased amount of acetone.2 These effects are impermanent. Hospitalization and time are able to cure the effects of acetone exposure.1
Conclusion
MTBE is no longer commonly used as a gasoline oxygenate. In 2005, reformulated gasoline was not required to contain oxygenates, and state and federal governments dramatically limited MTBE use. Future studies are needed to determine if MTBE is still present in groundwater used for public consumption. Leaking tanks should be addressed and gasoline spills should be studied with care. Despite the fact that MTBE does not pose a life-threatening risk, it is still a contaminant that should be eradicated quickly for the public good. Furthermore, alternatives to MTBE like ethanol should be explored and used when an oxygenate is needed for octane improvement.
References
- Agency for Toxic Substances and Disease Registry. May, 2014. “Health Effects.” 1-152. Retrieved from http://www.atsdr.cdc.gov/ToxProfiles/tp91-c2.pdf
- John T. Wilson & Philip M. Kaiser, et al. January, 2005. “Monitored Natural Attenuation of MTBE as a Risk Management Option at Leaking Underground Storage Tank Sites.” Retrieved from http://bit.ly/2tdow7y
- New Energy and Fuel. October, 2012. “New Energy and Fuel.” Evonik Plant Photo. Image. Retrieved from http://bit.ly/2rTmGXY
- United States Environmental Protection Agency. May, 2013. “Methyl Tertiary Butyl Ether (MTBE).” United States Environmental Protection Agency. Retrieved from http://www.epa.gov/mtbe/faq.htm
- United States Geologic Survey. February, 2013. “Aerobic Biodegradation.” Retrieved from http://toxics.usgs.gov/definitions/aerobic_biodegradation.html
- U.S. Government Printing Office. May 21, 2002. “MTBE Contamination in Groundwater: Identifying and Addressing the Problem.” U.S. Government Printing Office, 1-82. Retrieved from
http://bit.ly/2vm5BZ9

